Thermal Expansion case study
Introduction
When heat is applied to most materials, expansion occurs in all directions. Conversely, if heat energy is removed from a material (i.e. the material is cooled) contraction occurs in all directions. The effects of expansion and contraction each depend on the change of temperature of the material.
Coefficient of Linear Expansion
The amount by which unit length of a material expands when the temperature is raised one degree is called the coefficient of linear expansion of the material and is represented by (𝛼)
the coefficient of linear expansion means that a 1 m long material expands by (dl ) m if its temperature is increased by 1 K (or 1°C).
If a material, initially of length l1 and at a temperature of t1 and having a coefficient of linear expansion 𝛼, has its temperature increased to t2, then the new length l2 of the material is given by:
If a material having an initial surface area A1 at temperature t1 and having a coefficient of superficial expansion 𝛽, has its temperature increased to t2, then the new surface area A2 of the material is given by:
It may be shown that the coefficient of superficial expansion is twice the coefficient of linear expansion, i.e. 𝛽= 2𝛼 , to a very close approximation.
Coefficient of Cubic Expansion
The amount by which unit volume of a material increases for a one degree rise of temperature is called the coefficient of cubic (or volumetric) expansion and is represented by 𝛄
If a material having an initial volume V1 at temperature t1 and having a coefficient of cubic expansion 𝛄, has its temperature raised to t2, then the new volume V2 of the material is given by:
It may be shown that the coefficient of cubic expansion is three times the coefficient of linear expansion, i.e.𝛄 = 3 𝛼 , to a very close approximation.
A liquid has no definite shape and only its cubic or volumetric expansion need be considered. Thus with expansions in liquids
Practical Applications of Thermal Expansion
Some practical applications where expansion and contraction of solid materials
must be allowed for include:
(i) Overhead electrical transmission lines are hung so that they are slack in summer, otherwise their contraction in winter may snap the conductors or bring down pylons.
(ii) Gaps need to be left in lengths of railway lines to prevent buckling in hot weather (except where these are continuously welded).
(iii) Ends of large bridges are often supported on rollers to allow them to expand and contract freely.
(iv) Fitting a metal collar to a shaft or a steel tyre to a wheel is often achieved by first heating them so that they expand, fitting them in position, and then cooling them so that the contraction holds them firmly in place; this is known as a ‘shrink-fit’. By a similar method hot rivets are used for joining metal sheets.
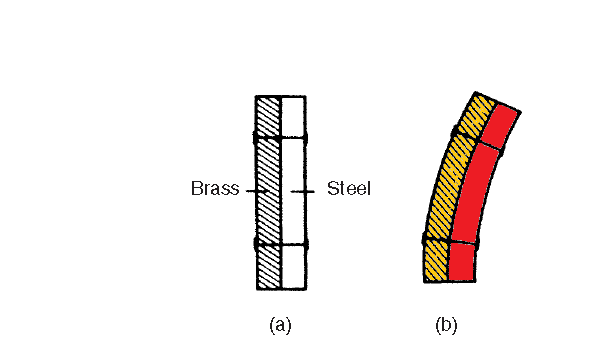
(v) The amount of expansion varies with different materials. Figure (a) shows a bimetallic strip at room temperature (i.e. two different strips of metal riveted together). When heated, brass expands more than steel, and since the two metals are riveted together the bimetallic strip is forced into
an arc as shown in Figure (b). Such a movement can be arranged to make or break an electric circuit and bimetallic strips are used, in particular, in thermostats (which are temperature-operated switches) used to control central heating systems, cookers, refrigerators, toasters, irons, hot water and alarm systems.
(vi) Motor engines use the rapid expansion of heated gases to force a piston to move.
(vii) Designers must predict, and allow for, the expansion of steel pipes in a steam-raising plant so as to avoid damage and consequent danger to health.
Expansion and Contraction of Water
Water is a liquid that at low temperature displays an unusual effect. If cooled, contraction occurs until, at about 4°C, the volume is at a minimum. As the temperature is further decreased from 4°C to 0°C expansion occurs, i.e. the volume increases. When ice is formed, considerable expansion occurs and it is this expansion that often causes frozen water pipes to burst.
A practical application of the expansion of a liquid is with thermometers, where the expansion of a liquid, such as mercury or alcohol, is used to measure temperature.
Thermal expansion coefficients
Linear temperature expansion coefficients for aluminum, copper, glass, iron and other common materials
When an object is heated or cooled, its length change by an amount proportional to the original length and the change in temperature
Thermal expansion coefficients for some common materials
m/m = meter per meter, in/in = inches per inches
tK = tC + 273.16
tR = tF + 459.67
1 in (inch) = 25.4 mm
1 ft (foot) = 0.3048 m
Introduction
When heat is applied to most materials, expansion occurs in all directions. Conversely, if heat energy is removed from a material (i.e. the material is cooled) contraction occurs in all directions. The effects of expansion and contraction each depend on the change of temperature of the material.
Coefficient of Linear Expansion
The amount by which unit length of a material expands when the temperature is raised one degree is called the coefficient of linear expansion of the material and is represented by (𝛼)
the coefficient of linear expansion means that a 1 m long material expands by (dl ) m if its temperature is increased by 1 K (or 1°C).
If a material, initially of length l1 and at a temperature of t1 and having a coefficient of linear expansion 𝛼, has its temperature increased to t2, then the new length l2 of the material is given by:
Coefficient of Superficial Expansion
The amount by which unit area of a material increases when the temperature is raised by one degree is called the coefficient of superficial (i.e. area) expansion and is represented by 𝛽If a material having an initial surface area A1 at temperature t1 and having a coefficient of superficial expansion 𝛽, has its temperature increased to t2, then the new surface area A2 of the material is given by:
A2 = A1 [1 + 𝛽.(t2 − t1)]
It may be shown that the coefficient of superficial expansion is twice the coefficient of linear expansion, i.e. 𝛽= 2𝛼 , to a very close approximation.
Coefficient of Cubic Expansion
The amount by which unit volume of a material increases for a one degree rise of temperature is called the coefficient of cubic (or volumetric) expansion and is represented by 𝛄
If a material having an initial volume V1 at temperature t1 and having a coefficient of cubic expansion 𝛄, has its temperature raised to t2, then the new volume V2 of the material is given by:
V2 = V1[1+ 𝛄.(t2 − t1)]
It may be shown that the coefficient of cubic expansion is three times the coefficient of linear expansion, i.e.𝛄 = 3 𝛼 , to a very close approximation.
A liquid has no definite shape and only its cubic or volumetric expansion need be considered. Thus with expansions in liquids
Practical Applications of Thermal Expansion
Some practical applications where expansion and contraction of solid materials
must be allowed for include:
(i) Overhead electrical transmission lines are hung so that they are slack in summer, otherwise their contraction in winter may snap the conductors or bring down pylons.
(ii) Gaps need to be left in lengths of railway lines to prevent buckling in hot weather (except where these are continuously welded).
(iii) Ends of large bridges are often supported on rollers to allow them to expand and contract freely.
(iv) Fitting a metal collar to a shaft or a steel tyre to a wheel is often achieved by first heating them so that they expand, fitting them in position, and then cooling them so that the contraction holds them firmly in place; this is known as a ‘shrink-fit’. By a similar method hot rivets are used for joining metal sheets.
(v) The amount of expansion varies with different materials. Figure (a) shows a bimetallic strip at room temperature (i.e. two different strips of metal riveted together). When heated, brass expands more than steel, and since the two metals are riveted together the bimetallic strip is forced into
an arc as shown in Figure (b). Such a movement can be arranged to make or break an electric circuit and bimetallic strips are used, in particular, in thermostats (which are temperature-operated switches) used to control central heating systems, cookers, refrigerators, toasters, irons, hot water and alarm systems.
(vi) Motor engines use the rapid expansion of heated gases to force a piston to move.
(vii) Designers must predict, and allow for, the expansion of steel pipes in a steam-raising plant so as to avoid damage and consequent danger to health.
Expansion and Contraction of Water
Water is a liquid that at low temperature displays an unusual effect. If cooled, contraction occurs until, at about 4°C, the volume is at a minimum. As the temperature is further decreased from 4°C to 0°C expansion occurs, i.e. the volume increases. When ice is formed, considerable expansion occurs and it is this expansion that often causes frozen water pipes to burst.
A practical application of the expansion of a liquid is with thermometers, where the expansion of a liquid, such as mercury or alcohol, is used to measure temperature.
Thermal expansion coefficients
Linear temperature expansion coefficients for aluminum, copper, glass, iron and other common materials
When an object is heated or cooled, its length change by an amount proportional to the original length and the change in temperature
Thermal expansion coefficients for some common materials
Product
|
Linear Temperature ExpansionCoefficient
- α -
10-6 (m/(m K))
|
|---|---|
ABS (Acrylonitrile butadiene styrene) thermoplastic
|
72 - 108
40 - 60 |
ABS -glass fiber-reinforced
|
31
17.2 |
Acetals
|
85 - 110
47.2 - 61.1 |
Acetal - glass fiber-reinforced
|
39
21.7 |
Acrylic
|
68 - 75
37.8 - 41.7 |
Alumina (aluminium oxide, Al2O3)
|
8.1
4.5 |
Aluminum
|
21 - 24
11.7 - 13.3 |
Aluminum nitride
|
5.3
2.94 |
Amber
|
50 - 60
27.8 - 33.3 |
Antimony
|
9 - 11
5 - 6.11 |
Arsenic
|
4.7
2.61 |
Bakelite, bleached
|
22
12.2 |
Barium
|
20.6
11.4 |
Barium ferrite
|
10
5.56 |
Benzocyclobutene
|
42
23.3 |
Beryllium
|
12
6.67 |
Bismuth
|
13 - 13.5
7.22 - 7.5 |
Brass
|
18 - 19
10 - 10.6 |
Brick masonry
|
5
2.78 |
Bronze
|
17.5 - 18
9.72 - 10 |
Cadmium
|
30
16.7 |
Calcium
|
22.3
12.4 |
Cast Iron Gray
|
10.8
6 |
Caoutchouc
|
66 - 69
36.7 - 38.3 |
Celluloid
|
100
55.6 |
Cellulose acetate (CA)
|
130
72.2 |
Cellulose acetate butynate (CAB)
|
96 - 171
53.3 - 95 |
Cellulose nitrate (CN)
|
80 - 120
44.4 - 66.7 |
Cement, Portland
|
11
6.11 |
Cerium
|
5.2
2.89 |
Chlorinated polyether
|
80
44.4 |
Chlorinated polyvinylchloride (CPVC)
|
63 - 66
35 - 36.7 |
Chromium
|
6 - 7
3.33 - 3.89 |
Clay tile structure
|
5.9
3.28 |
Cobalt
|
12
6.67 |
Concrete
|
13 - 14
7.22 - 7.78 |
Concrete structure
|
9.8
5.44 |
Constantan
|
15.2 - 18.8
8.44 - 10.4 |
Copper
|
16 - 16.7
8.89 - 9.28 |
Copper, Beryllium 25
|
17.8
9.89 |
Corundum, sintered
|
6.5
3.61 |
Cupronickel 30%
|
16.2
9 |
Diamond (Carbon)
|
1.1 - 1.3
0.61 - 0.72 |
Duralumin
|
23
12.8 |
Dysprosium
|
9.9
5.5 |
Ebonite
|
70
38.9 |
Epoxy, cast resins & compounds, unfilled
|
45 - 65
25 - 36.1 |
Epoxy - glass fiber reinforced
|
36
20 |
Erbium
|
12.2
6.78 |
Ethylene ethyl acrylate (EEA)
|
205
114 |
Ethylene vinyl acetate (EVA)
|
180
100 |
Europium
|
35
19.4 |
Fluoroethylene propylene (FEP)
|
135
75 |
Fluorspar, CaF2
|
19.5
10.8 |
Gadolinium
|
9
5 |
Germanium
|
6.1
3.39 |
German silver
|
18.4
10.2 |
Glass, hard
|
5.9
3.28 |
Glass, Pyrex
|
4.0
2.22 |
Glass, plate
|
9.0
5 |
Gold
|
14.2
7.89 |
Gold - copper
|
15.5
8.61 |
Gold - platinum
|
15.2
8.44 |
Granite
|
7.9 - 8.4
4.39 - 4.67 |
Graphite, pure (Carbon)
|
4 -8
2.22 |
Gunmetal
|
18
10 |
Gutta percha
|
198
110 |
Hafnium
|
5.9
3.28 |
Hard alloy K20
|
6
3.33 |
Hastelloy C
|
11.3
6.28 |
Holmium
|
11.2
6.22 |
Ice, 0oC water
|
51
28.3 |
Inconel
|
11.5 - 12.6
6.39 - 7 |
Indium
|
33
18.3 |
Invar
|
1.5
0.83 |
Iridium
|
6.4
3.56 |
Iron, pure
|
12.0
6.67 |
Iron, cast
|
10.4 - 11
5.78 - 6.11 |
Iron, forged
|
11.3
6.28 |
Kapton
|
20
11.1 |
Lanthanum
|
12.1
6.72 |
Lead
|
29
16.1 |
Limestone
|
8
4.44 |
Lithium
|
46
25.6 |
Lutetium
|
9.9
5.5 |
Macor
|
9.3
5.17 |
Magnalium
|
23.8
13.2 |
Magnesium
|
25 - 26.9
13.9 - 14.9 |
Manganese
|
22
12.2 |
Manganin
|
18.1
10.1 |
Marble
|
5.5 - 14.1
3.06 - 7.83 |
Masonry, brick
|
4.7 - 9.0
2.61 - 5 |
Mercury
|
61
33.9 |
Mica
|
3
1.67 |
Molybdenum
|
5
2.78 |
Monel metal
|
13.5
7.5 |
Mortar
|
7.3 - 13.5
4.06 - 7.5 |
Neodymium
|
9.6
5.33 |
Nickel
|
13.0
7.22 |
Niobium (Columbium)
|
7
3.89 |
Nylon, general purpose
|
50 - 90
27.8 - 50 |
Nylon, glass fiber reinforced
|
23
12.8 |
Nylon, Type 11, molding and extruding compound
|
100
55.6 |
Nylon, Type 12, molding and extruding compound
|
80.5
44.7 |
Nylon, Type 6, cast
|
85
47.2 |
Nylon, Type 6/6, molding compound
|
80
44.4 |
Oak, perpendicular to the grain
|
54
30 |
Osmium
|
5 - 6
2.78 - 3.33 |
Palladium
|
11.8
6.56 |
Paraffin
|
106 - 480
58.9 - 267 |
Phenolic resin without fillers
|
60 - 80
33.3 - 44.4 |
Phosphor bronze
|
16.7
9.28 |
Plaster
|
17
9.44 |
Plastics
|
40 - 120
22.2 - 66.7 |
Platinum
|
9
5 |
Plutonium
|
47 - 54
26.1 - 30 |
Polyacrylonitrile
|
70
38.9 |
Polyallomer
|
92
51.1 |
Polyamide (PA)
|
110
61.1 |
Polybutylene (PB)
|
130 - 139
72.2 - 77.2 |
Polycarbonate (PC)
|
65 - 70
36.1 - 38.9 |
Polycarbonate - glass fiber-reinforced
|
21.5
11.9 |
Polyester
|
124
68.9 |
Polyester - glass fiber-reinforced
|
25
13.9 |
Polyethylene (PE)
|
108 - 200
60 - 111 |
Polyethylene (PE) - High Molecular Weight
|
108
60 |
Polyethylene terephthalate (PET)
|
59.4
33 |
Polyphenylene
|
54
30 |
Polyphenylene - glass fiber-reinforced
|
36
20 |
Polypropylene (PP), unfilled
|
72 - 90
40 - 50 |
Polypropylene - glass fiber-reinforced
|
32
17.8 |
Polystyrene (PS)
|
70
38.9 |
Polysulfone (PSO)
|
55 - 60
30.6 - 33.3 |
Polytetrafluorethylene (PTFE)
|
112 - 135
62.2 - 75 |
Polyurethane (PUR), rigid
|
57.6
32 |
Polyvinyl chloride (PVC)
|
54 - 110
30 - 61.1 |
Polyvinylidene fluoride (PVDF)
|
128 - 140
71.1 - 77.8 |
Porcelain, Industrial
|
4
2.22 |
Potassium
|
83
46.1 |
Praseodymium
|
6.7
3.72 |
Promethium
|
11
6.11 |
Quartz, mineral
|
8 - 14
4.44 - 7.78 |
Quartz, fused
|
0.55
0.31 |
Rhenium
|
6.7
3.72 |
Rhodium
|
8
4.44 |
Rock salt
|
40.4
22.4 |
Rubber, hard
|
80
44.4 |
Ruthenium
|
9.1
5.06 |
Samarium
|
12.7
7.06 |
Sandstone
|
11.6
6.44 |
Sapphire
|
5.3
2.94 |
Scandium
|
10.2
5.67 |
Selenium
|
37
20.6 |
Silicon
|
3 - 5
1.67 - 2.78 |
Silicon Carbide
|
2.77
1.54 |
Silver
|
19 - 19.7
10.6 - 10.9 |
Sitall
|
0.15
0.0833 |
Slate
|
10
5.56 |
Sodium
|
70
38.9 |
Solder lead - tin, 50% - 50%
|
25
13.9 |
Speculum metal
|
19.3
10.7 |
Steatite
|
8.5
4.72 |
Steel
|
11 - 12.5
6.11 - 6.94 |
Steel Stainless Austenitic (304)
|
17.3
9.61 |
Steel Stainless Austenitic (310)
|
14.4
8 |
Steel Stainless Austenitic (316)
|
16.0
8.89 |
Steel Stainless Ferritic (410)
|
9.9
5.5 |
Strontium
|
22.5
12.5 |
Tantalum
|
6.5
3.61 |
Tellurium
|
36.9
20.5 |
Terbium
|
10.3
5.72 |
Terne
|
11.6
6.44 |
Thallium
|
29.9
16.6 |
Thorium
|
12
6.67 |
Thulium
|
13.3
7.39 |
Tin
|
20 - 23
11.1 - 12.8 |
Titanium
|
8.5 - 9
4.72 - 5 |
Topas
|
5 - 8
2.78 - 4.44 |
Tungsten
|
4.5
2.5 |
Uranium
|
13.4
7.44 |
Vanadium
|
8
4.44 |
Vinyl Ester
|
16 - 22
8.89 - 12.2 |
Vulcanite
|
63.6
35.3 |
Wax
|
2 - 15
1.11 - 8.33 |
Wedgwood ware
|
8.9
4.94 |
Wood, fir
|
3.7
2.06 |
Wood, parallel to grain
|
3
1.67 |
Wood, across (perpendicular) to grain
|
30
16.7 |
Wood, pine
|
5
2.78 |
Ytterbium
|
26.3
14.6 |
Yttrium
|
10.6
5.89 |
Zinc
|
30 - 35
16.7 - 19.4 |
Zirconium
|
5.7
3.17 |
Most values for temperature 25 C (77 F). The span in the values may be caused by the variation in the materials themselves - or by the variation in the sources used





No comments:
Post a Comment
thanks for your visit