Properties of Pressure,Measurement AND gauges Case Study
Introductionpressure is the force exerted by a fluid per unit area.
P = F/A.
A fluid has a negligible resistance to a shear force, so that the force it exerts always acts at right angles to its containing surface. |
| FIG 1 |
The SI unit of pressure is the pascal, Pa, which is unit force per unit area, i.e. 1 Pa = 1 N/m² The pascal is a very small unit and a commonly used larger unit is the bar, where 1 bar = 105 Pa
Atmospheric pressure is due to the mass of the air above the earth’s surface figure 2 . Atmospheric pressure changes continuously. A standard value of atmospheric pressure, called ‘standard atmospheric pressure
 |
| FIG 1 |
unit, the millibar, is usually used in the measurement of meteorological pressures. (Note that when atmospheric pressure varies from 101 325 Pa it is no longer standard.)
Properties of Pressure in a Fluid
There are three important observations about pressure in a fluid.
1) At any point that a fluid is in contact with a surface, the pressure is directed perpendicular to the surface.
2) At any point inside a fluid, the pressure is directed in all directions with the same magnitude. (See figure 2). For the block in this figure, the pressure is not exactly the same because the different faces are at different depths. But if the block is infinitesimally small, then the pressure in all directions is exactly the same.
3) The pressure at any point in a fluid depends only on the depth of the point. Suppose there is a volume of fluid with a uniform density which has a depth h and area A.
There is the force from the atmosphere above the liquid F0 = P0×A. There is the weight of the liquid Mg. There is also the force of the liquid pushing up on the column of liquid at depth h.
Since there is no acceleration of the liquid at the depth h, the forces must balance each other.
∑Fy = 0
PA -Mg - P0A = 0
PA -𝜌Vg - P0A = 0
PA -𝜌hAg - P0A = 0
P = 𝜌gh +P0
where:
𝜌 : fluid density (kg/m³)
g : acceleration of gravity (m/sec²)
So the pressure at any depth h in a fluid is equal to the pressure outside of the fluid (P0) plus the fluid pressure (𝜌hg).
For many circumstances, the pressure outside of the fluid is the pressure of the earth’s atmosphere at that point. For instance, the pressure in your tires is really the pressure of the air in your tires plus the pressure of the atmosphere on the tires. However, the pressure that is read on a pressure gauge is the pressure which is greater than the atmospheric pressure. It is called gauge pressure. In general, the equation above becomes
P = PG +PA
where
PG is the gauge pressure
PA is the atmospheric pressure.
Pascal’s Principle
Pascal’s principle states that pressure applied to a confined fluid increases the pressure throughout the fluid by the same amount.
This is how a hydraulic lift works. Fluid is enclosed in a pipe with a small area at one end and a large area at the other. Pressure is applied at the end with the small area. That same pressure is transferred to the end with the large area.
P1 = F1 /A1 = P2 = F2 /A2
F2 = F1 (A2 /A1)
If the area A2 is much larger than the area A1, then a small force F1 can be applied to create a large force F2 at the output end. This large force can be used to jack up a car or lift heavy objects.
Measurements of Pressure
Pressure is measured using the two principles P =𝜌gh, and Pascal’s Principle.
The pressure at any height is equal, so the pressure of the atmosphere, just equals the pressure of the liquid or 𝜌gh
Pressure Indicators Types
(i) barometers
(ii) manometers
(iii) Bourdon pressure gauge
(iv) McLeod and Pirani gauges
Barometers
A barometer is an instrument for measuring atmospheric pressure. It is affected by seasonal changes of temperature. Barometers are therefore also used for the measurement of altitude and also as one of the aids in weather forecasting. The value of atmospheric pressure will thus vary with climatic conditions, although not usually by more than about 10% of standard atmospheric pressure.
Construction and principle of operation
A simple barometer consists of a glass tube, just under 1 m in length, sealed at one end, filled with mercury and then inverted into a trough containing more mercury. Care must be taken to ensure that no air enters the tube during this latter process. Such a barometer is shown in Figure 3 and it is seen that the level of the mercury column falls, leaving an empty space, called a vacuum. Atmospheric pressure acts on the surface of the mercury in the trough
 |
| FIG 3 |
atmospheric pressure, i.e. a vertical column of mercury 760 mm high exerts a pressure equal to the standard value of atmospheric pressure.
There are thus several ways in which atmospheric pressure can be expressed:
Standard atmospheric pressure = 101 325 Pa or 101.325 kPa
= 101 325 N/m² or 101.325 kN/m²
= 1.01325 bars or 1013.25 mbars
=760 mm of mercury
Another arrangement of a typical barometer is shown in Figure 4 where a U-tube is used instead of an inverted tube and trough, the principle being similar. If, instead of mercury, water was used as the liquid in a barometer, then the barometric height h at standard atmospheric pressure would be 13.6 times more than for mercury, i.e. about 10.4 m high, which is not very practicable. This is because the relative density of mercury is 13.6
 |
| FIG 4 |
1- The Fortin barometer
 |
| FIG 5 |
2- the aneroid barometer
 |
| FIG 6 |
Absolute and Gauge Pressure
The term absolute pressure means the pressure above that of an absolute vacuum (which is zero pressure). In Figure 7 . a pressure scale is shown with the line AB representing absolute zero pressure (i.e. a vacuum) and line CD representing atmospheric pressure. With most practical pressure-measuring instruments the part of the instrument that is subjected to the pressure being measured is also subjected to atmospheric pressure. Thus practical instruments actually determine the difference between the pressure being measured and atmospheric pressure. The pressure that the instrument is measuring is then termed the gauge pressure. In Figure 7 , the line EF represents an absolute pressure that has a value greater than atmospheric pressure, i.e. the ‘gauge’ pressure is positive. Thus,
 |
| FIG 7 |
absolute pressure = gauge pressure + atmospheric pressure
Pressure-measuring indicating instruments are referred to generally as pressure gauges (which acts as a reminder that they measure ‘gauge’ pressure).
for the pressure indicated on a pressure gauge to be below atmospheric pressure, i.e. the gauge pressure is negative. Such a gauge pressure is often referred to as a vacuum, even though it does not necessarily represent a complete vacuum at absolute zero pressure. The line GH in Figure 7 shows such a pressure. An indicating instrument used for measuring such pressures is called a vacuum gauge.
example : A vacuum gauge indication of, say, 0.4 bar means that the pressure is 0.4 bar less than atmospheric pressure. If atmospheric pressure is 1 bar, then the absolute pressure is 1–0.4 or 0.6 bar.
The Manometer
A manometer is a device for measuring or comparing fluid pressures, and is the simplest method of indicating such pressures.
1- U-tube manometer
A U-tube manometer consists of a glass tube bent into a U shape and containing a liquid such as mercury. A U-tube manometer is shown in Figure 8 (a). If limb A is connected to a container of gas whose pressure is above atmospheric, then the pressure of the gas will cause the levels of mercury to move as shown in Figure 8 (b), such that the difference in height is h1. The measuring scale can be calibrated to give the gauge pressure of the gas as h1 mm of mercury.
 |
| FIG 8 |
If limb A is connected to a container of gas whose pressure is below atmospheric then the levels of mercury will move as shown in Figure 8 (c), such that their pressure difference is h2 mm of mercury.
It is also possible merely to compare two pressures, say, PA and PB, using a U-tube manometer. Figure 8 (d) shows such an arrangement with
(PB - PA) equivalent to h mm of mercury. One application of this differential pressure-measuring device is in determining the velocity of fluid flow in pipes
For the measurement of lower pressures, water or paraffin may be used instead of mercury in the U-tube to give larger values of h and thus greater sensitivity.
2- Inclined manometers
 |
| FIG 9 |
Pressures many times greater than atmospheric can be measured by the Bourdon pressure gauge, which is the most extensively used of all pressure indicating instruments
 |
| FIG 10 |
It is a robust instrument. Its main component is a piece of metal tube (called the Bourdon tube), usually made of phosphor bronze or alloy steel, of oval or elliptical cross-section, sealed at one end and bent into an arc. In some forms the tube is bent into a spiral for greater sensitivity. A typical arrangement is shown in Figure 10 (a). One end, E, of the Bourdon tube is fixed and the fluid whose pressure is to be measured is connected to this end. The pressure acts at right angles to the metal tube wall as shown in the cross-section of the tube in Figure 10 (b). Because of its elliptical shape
it is clear that the sum of the pressure components, i.e. the total force acting on the sides A and C, exceeds the sum of the pressure components acting on ends B and D. The result is that sides A and C tend to move outwards and B and D inwards tending to form a circular cross-section. As the pressure in the tube is increased the tube tends to uncurl, or if the pressure is reduced the tube curls
up further. The movement of the free end of the tube is, for practical purposes, proportional to the pressure applied to the tube, this pressure, of course, being the gauge pressure (i.e. the difference between atmospheric pressure acting on the outside of the tube and the applied pressure acting on the inside of the tube). By using a link, a pivot and a toothed segment as shown in Figure 10 (a), the movement can be converted into the rotation of a pointer over a graduated calibrated scale.
The Bourdon tube pressure gauge is capable of measuring high pressures up to 104 bar (i.e. 7600 m of mercury) with the addition of special safety features. A pressure gauge must be calibrated, and this is done either by a manometer, for low pressures, or by a piece of equipment called a ‘dead weight tester’. This tester consists of a piston operating in an oil-filled cylinder of known bore, and carrying accurately known weights as shown in Figure 11 . The gauge under test is attached to the tester and a screwed piston or ram applies the required pressure until the weights are just lifted. While the gauge is being read, the weights are turned to reduce friction effects.
 |
| FIG 11 |
Vacuum gauges are instruments for giving a visual indication, by means of a pointer, of the amount by which the pressure of a fluid applied to the gauge is less than the pressure of the surrounding atmosphere. Two examples of vacuum gauges are the McLeod gauge and the Pirani gauge
1- McLeod gauge
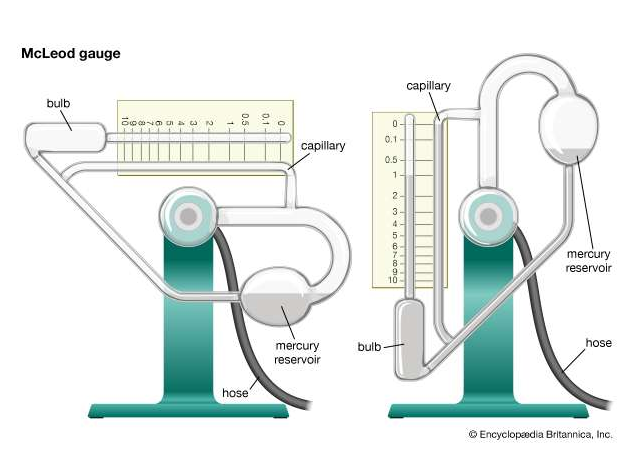 |
| FIG 12 |
2- Pirani gauge
 |
| FIG 13 |



This blog seems sharing interesting tips, as I was looking to buy Voltage Output Pressure Transducers and other equipments.
ReplyDeletedigital temperature indicator with sensor
The content highlights important considerations effectively; consulting Pressure Gauges may provide further relevant information.
ReplyDelete