Corrosion prevention by Cathodic protection
INTRODUCTION
Cathodic protection. A means of corrosion prevention whereby electrons are supplied to the structure
to be protected from an external source such as another more reactive metal or a dc power supply.
CATHODIC PROTECTION
Use of cathodic protection to reduce both interior and exterior corrosion is controversial and complex. Industry experience shows that it is very useful for interior corrosion on crude oil tanks when used in conjunction with liners. However, cathodic protection has not been proven universally effective for protecting finished fuel tanks from internal corrosion. Coatings do that job adequately. On external or underside corrosion, cathodic protection has been used with mixed results. Theoretically, cathodic protection will work if installed properly, but in reality there are many obstacles to overcome for it to work right. Unless these systems are installed, tested, maintained,
and operated by trained and qualified people, they can be totally ineffective and, in fact, can cause accelerated corrosion. Cathodic protection should not be mandated as a blanket solution but should be evaluated and weighed individually against other alternatives on a site-specific basis
ELECTROCHEMICAL PRINCIPLES
Cathodic protection is an electrochemical technique in which a cathodic (protective) potential is applied to an engineering structure in order to prevent corrosion from taking place. This implies that Ohm’s law, E = IR, can be used to control the potential, as well as the current. Hence, metal oxidation is prevented since the potential must be below the corrosion potential the main reason for this potential-control technique. In principle, all structures
This is can be protected cathodically, but structural steels being the most common ferrous materials used to build large structures are cathodically protected by an external potential
According to the electrochemical principles, the formation of a solid oxide corrosion product on a metal M immersed in an electrolyte depends on the constituents in solution. For instance, consider the presence and absence of oxygen in solution. The following reactions for a hypothetical oxidizing metal M are used to classify the type of solution
Thus, an aerated solution contains oxygen and its deaerated counterpart lacks of dissolved oxygen. The former is the most common reaction encounter in industrial schemes. For corroding iron or steel, the coupled anodic and cathodic reactions in the presence of oxygen generate an overall (redox) reaction similar to eq. above, which is formed as follows
The principles for corrosion and cathodic protection is illustrated in Figure 1 for an iron or carbon steel structure. Corrosion occurs at a slow or fast rate of iron dissolution in the aerated electrolyte since it is the iron atoms that release electrons, which are needed for the reduction of water to form hydroxylions in the electrolyte, such as air or soil. On the other hand, cathodic protection is achieved by supplying external electrons to the structure. Thus, the amount of external electrons reduce significantly or prevent the rate of dissolution of iron, but hydroxylions still form on the structure surface.
 |
| FIG 1 |
APPLICATIONS
- natural gas propane piping;
- liquid fuel piping;
- oxygen piping;
- underground storage tanks;
- fire protection piping;
- ductile iron pressurized piping under floor (slab on grade) in soil
- underground heat distribution and chilled water piping in ferrous metallic conduit in soils with resistivity of 30,000 ohm-cm or less; and
- other structures with hazardous products as identified by the user of the facility.
IMPRESSED CURRENT TECHNIQUE
The impressed current technique is a simple and yet, significant form of cathodic protection of underground steel pipelines as shown in Figure 2. A buried pipeline is connected to the positive terminal of a rectifier (power supply) and the anode to the negative terminal. Both terminals must be well insulated; otherwise, current leakage (stray-current) occurs and the structure may not be protected adequately. The significance of the cathodic protection setups shown in Figure 2,3 is that the current flows from the rectifier to the inert anode or sacrificial anode (graphite) through the soil (electrolyte) to the cathode. For structures immersed in seawater, the anodes may be platinum-coated titanium or high-silicon cast iron . The purpose of the rectifier is to convert alternating current (ac) to uniform direct current (dc).
 |
| FIG 2 |
 |
| FIG 3 |
 |
| FIG 4 |
 |
| FIG 5 |
where
I = Ground current
D = Diameter of the column
h = Length of the column
CATHODIC PROTECTION DESIGN
The following steps and information is required to ensure a cathodic protection system will perform as designed:
Step 1. Collect data:
- corrosion history of similar piping in the area
- drawings
- tests to include current requirement, potential survey and soil resistivity survey
- life of structures to be protected
- coatings
- short circuits.
Step 2. Calculate the surface area to be protected and determine the current requirement.
Step 3. Select the anode type and calculate the number of anodes required
Step 4. Calculate circuit resistance, required voltage, and current.
Step 5. Prepare life cycle cost analyses.
Step 6. Prepare plans and specifications




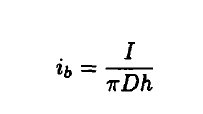
No comments:
Post a Comment
thanks for your visit