An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeters
Introduction
Calorimetry is the field of science that deals with the measurement of the state of a body with respect to the thermal aspects in order to examine its physical and chemical changes. The changes could be physical such as melting, evaporation etc or could also be chemical such as burning, acid-base neutralization etc. Calorimeter is what is used to measure the thermal changes of a body. Calorimetry is applied extensively in the fields of thermochemistry in calculating the enthalpy, stability, heat capacity etc.

Types of Calorimeter
The different types of calorimeters are
1- Adiabatic Calorimeters
2- Reaction Calorimeters
3- Bomb Calorimeters (Constant Volume Calorimeters)
4- Constant Pressure Calorimeters
5- Differential Scanning Calorimeters
Adiabatic calorimeters
An adiabatic calorimeter is a calorimeter used to examine a runaway reaction. Since the calorimeter runs in an adiabatic environment, any heat generated by the material sample under test causes the sample to increase in temperature, thus fueling the reaction.
No adiabatic calorimeter is fully adiabatic - some heat will be lost by the sample to the sample holder. A mathematical correction factor, known as the phi-factor, can be used to adjust the calorimetric result to account for these heat losses. The phi-factor is the ratio of the thermal mass of the sample and sample holder to the thermal mass of the sample alone.
 |
| FIG 1 |
Reaction calorimeters
A reaction calorimeter is a calorimeter in which a chemical reaction is initiated within a closed insulated container. Reaction heats are measured and the total heat is obtained by integrating heatflow versus time. This is the standard used in industry to measure heats since industrial processes are engineered to run at constant temperatures.[citation needed] Reaction calorimetry can also be used to determine maximum heat release rate for chemical process engineering and for tracking the global kinetics of reactions. There are four main methods for measuring the heat in reaction calorimeter:
 |
| FIG 2 |
Constant-pressure calorimeter
A constant-pressure calorimeter measures the change in enthalpy of a reaction occurring in solution during which the atmospheric pressure remains constant.
An example is a coffee-cup calorimeter, which is constructed from two nested Styrofoam cups and a lid with two holes, allowing insertion of a thermometer and a stirring rod. The inner cup holds a known amount of a solvent, usually water, that absorbs the heat from the reaction. When the reaction occurs, the outer cup provides insulation. Then

where
 = Specific heat at constant pressur
= Specific heat at constant pressur = Enthalpy of solution
= Enthalpy of solution = Change in temperature
= Change in temperature = mass of solvent
= mass of solvent = molecular mass of solvent
= molecular mass of solvent |
| FIG 3 |
The measurement of heat using a simple calorimeter, like the coffee cup calorimeter, is an example of constant-pressure calorimetry, since the pressure (atmospheric pressure) remains constant during the process. Constant-pressure calorimetry is used in determining the changes in enthalpy occurring in solution. Under these conditions the change in enthalpy equals the heat.
Differential scanning calorimeter
In a differential scanning calorimeter (DSC), heat flow into a sample—usually contained in a small aluminium capsule or 'pan'—is measured differentially, i.e., by comparing it to the flow into an empty reference pan.
In a heat flux DSC, both pans sit on a small slab of material with a known (calibrated) heat resistance K. The temperature of the calorimeter is raised linearly with time (scanned), i.e., the heating rate dT/dt = β is kept constant. This time linearity requires good design and good (computerized) temperature control. Of course, controlled cooling and isothermal experiments are also possible.
Heat flows into the two pans by conduction. The flow of heat into the sample is larger because of its heat capacity Cp. The difference in flow dq/dt induces a small temperature difference ΔT across the slab. This temperature difference is measured using a thermocouple. The heat capacity can in principle be determined from this signal:
A modulated temperature differential scanning calorimeter (MTDSC) is a type of DSC in which a small oscillation is imposed upon the otherwise linear heating rate.
This has a number of advantages. It facilitates the direct measurement of the heat capacity in one measurement, even in (quasi-)isothermal conditions. It permits the simultaneous measurement of heat effects that respond to a changing heating rate (reversing) and that don't respond to the changing heating rate (non-reversing). It allows for the optimization of both sensitivity and resolution in a single test by allowing for a slow average heating rate (optimizing resolution) and a fast changing heating rate (optimizing sensitivity).
Safety Screening:- DSC may also be used as an initial safety screening tool. In this mode the sample will be housed in a non-reactive crucible (often Gold, or Gold plated steel), and which will be able to withstand pressure (typically up to 100 bar). The presence of an exothermic event can then be used to assess the stability of a substance to heat. However, due to a combination of relatively poor sensitivity, slower than normal scan rates (typically 2–3°/min - due to much heavier crucible) and unknown activation energy, it is necessary to deduct about 75–100 °C from the initial start of the observed exotherm to suggest a maximum temperature for the material. A much more accurate data set can be obtained from an adiabatic calorimeter, but such a test may take 2–3 days from ambient at a rate of 3 °C increment per half hour.
 |
| FIG 4 |
The bomb calorimeter
The bomb calorimeter is used to determine the calorific value of solid fuels and some of the less volatile liquid fuels such as fuel oil. It is also used by food technologists to determine the calorificvalue of foods. As with the fuels which we use, the foods which we eat are chemical combinations of carbon and hydrogen, and when the moisture content has been removed, they can be burned
like a fuel to release their stored energy.
There are several different designs of bomb calorimeter but basically the apparatus consists of a screw-topped pressure vessel, the bomb, surrounded by water in a lagged copper calorimeter. The
screw top of the bomb contains insulated electrical connections and a pressurising valve. This enables it to be charged with oxygen to ensure that there is sufficient for complete combustion of the fuel. A small quantity of water, about 10 ml, is also placed inside the bomb.
Its purpose is to ensure condensation of the steam formed during combustion and absorb any acid products which are formed. Leads from the electrical connections extend inside the bomb and one of them supports a small porcelain crucible containing a measured sample of fuel mf. For solid fuels the fuse were connected between the leads is positioned in contact with the fuel sample. With liquid fuels a cotton thread is hung from the fuse wire into the fuel sample. The water equivalent of the bomb and
copper calorimeter mwc is supplied by the makers of the apparatus.
The arrangement is shown in Figure 5. The assembled and pressurised bomb is placed inside the insulated copper calorimeter and the electrical leads are connected to a low-voltage power supply. A measured quantity of water mw is added to the calorimeter to above the level of the bomb but slightly
below the level of the electrical connections. The stirrer, insulated lid and sensitive thermometer are then placed in position. The apparatus is allowed to stand for a period of time to allow the temperature inside the calorimeter to stabilise.
 |
| FIG 5 |
This is done by plotting a graph of temperature against time, as shown in Figure 6.
 |
| FIG 6 |
The calorific value of the fuel can now be found by equating the heat released by the fuel to the heat received by the water, bomb and the copper calorimeter, that is
heat supplied by fuel - heat received by the water bomb and calorimeter
mf × CV - (mw + mwc) ×cw(T2 -T1)
CV = [(mw + mwc) ×cw(T2 -T1) ] / mf
Combustion of the hydrogen content of the fuel produces H₂O in the form of steam. This condenses inside the bomb, giving up its latent heat, which becomes a part of the total heat received by the
apparatus. Because this is included, the value of the calorific value obtained from the bomb calorimeter is known as the higher or gross calorific value of the fuel.
When a fuel is used in practical situations such as in an internal combustion engine, the steam from combustion leaves with the exhaust gases and condenses in the atmosphere or in the exhaust pipe. As a result its latent heat is not available for conversion into useful work and is subtracted from the higher calorific value of the fuel. This is then known as the lower or net calorific value. Fuel
technologists are able to calculate its value from a knowledge of the hydrogen content and it is the lower calorific value which is usually quoted by oil and gas suppliers.
 |
| FIG 7 |
Boy’s gas calorimeter
Boy’s gas calorimeter is used to determine the calorific value of gaseous fuels. It contains a burner from which the hot gases pass over a set of cooling tubes. A controlled flow of water through the
tubes cools the hot gases down to the gas supply temperature and thus extracts all the heat which has been released during combustion.
The burner receives a metered flow of gas at a steady supply pressure and a drain at the base of the calorimeter enables the condensed steam, formed from the combustion of hydrogen, to be
collected. The arrangement is shown in Figure 8 . Having adjusted the cooling water flow rate so that the exhaust gases emerge at approximately the ambient temperature, the cooling water inlet and outlet temperatures Ti and To are recorded. The gas supply pressure hg measured in metres of water on the U-tube manometer is also recorded together with the gas supply and exhaust temperature Ts. A gas meter reading is then taken and simultaneously
the measuring jars are placed in position to collect the cooling water and condensate.After a period of around 5 min the gas meter reading is again taken and the volume of gas Vs which has been
supplied is calculated. The mass of cooling water mw and the mass of condensate mc which have been collected are also recorded.
 |
| FIG 8 |
from metres of water into pascals, that is,
pg=hg 𝜌 g
pg = metres of water into pascals
hg = gas supply pressure
𝜌 = water density
g = gravity acceleration
This of course is the gauge pressure of the gas and it must now be converted to absolute supply pressure ps by adding to it the prevailing value of atmospheric pressure pa obtained from a Fortin or
precision aneroid barometer, that is,
ps=pg+ pa
The next task is to convert the volume of gas which has been used to the volume which it would occupy at NTP. You will recall that normal temperature, Tn - 15⁰C, and normal pressure,
pn - 101:325 kPa. This is done using the general gas equation
The higher or gross calorific value of the gas can now be calculated by equating the heat released by the gas to the heat received by the cooling water
heat released by gas - heat received by cooling water
The lower or net calorific value of the gas can be found by subtracting the latent heat given up by the condensate from the heat received by the cooling water:The value of the specific latent heat of vaporisation at NTP can be taken to be hfg -2453 kJ Kg⁻¹.
The cooling water flow rate through Boy’s gas calorimeter must be adjusted so that the exhaust gases emerge approximately at the ambient temperature


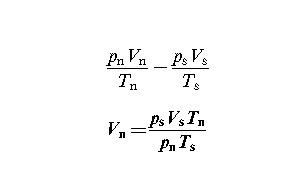



No comments:
Post a Comment
thanks for your visit